Leveraging a portfolio with purpose: Vertex in cystic fibrosis
In 2011, when Jeffrey Leiden joined Vertex Pharmaceuticals, he set an ambitious goal: to develop treatments for everyone suffering from cystic fibrosis (CF). Today Vertex is almost there, with a portfolio that can help up to 90% of patients (1).
"When I started at Vertex in 2011, I promised employees and patients that we would not stop until we had a treatment for nearly everyone with CF."
Dr Jeffrey Leiden, Former CEO, Vertex Pharmaceuticals, 2019 (2)
When we look across different industries, we often see how brands, portfolios and companies with a clear purpose tend to do better than others. It’s harder to talk purpose in Pharma though, as purpose is inherent in the product, but the Vertex story is a compelling one that we keep coming back to.
Here’s how we understand Vertex leveraged their CF portfolio to achieve a huge win for both themselves and for CF patients around the world.
Cystic fibrosis is a chronic rare disease caused by any of more than 1700 mutations to the CFTR gene, the most common of which is the F508del mutation (3).
Subscribe to our Pharma newsletter
There are a number of key challenges facing Pharma companies and we think you might value some insights into these challenges. Subscribe to get our latest articles in your Inbox. We won't spam you or share your email address with anyone else.
Whilst most treatments for CF only slow the decline in lung function over time, Vertex’s portfolio of drugs address the underlying mutations and allow for more effective control of the disease.
The recent introduction of Trikafta, Vertex’s latest addition to their growing CF portfolio, is particularly important in that it targets patients with just a single copy of the most common of the mutations (F508del). This has greatly expanded the number of patients who can now benefit from one of their brands.
So how has Vertex leveraged its portfolio and made a difference in the CF community?
There seem to be three key drivers:
- The long-term commitment to an ever-expanding portfolio
- Close relationships based on trust built directly with patients
- Innovative deals with payers to improve access to undeniably expensive treatments
First - an expanded portfolio means more CF patients than ever before are now eligible for Vertex’s drugs.
Across North America, Europe and Australia there are approximately 75,000 patients with CF (4); before the introduction of Trikafta, drugs from Vertex’s portfolio were already being used to treat around 60% of them (5). With the addition of Trikafta, a combination brand of three distinct entities, Vertex now has a portfolio of brands for which 90% of the CF population is eligible.
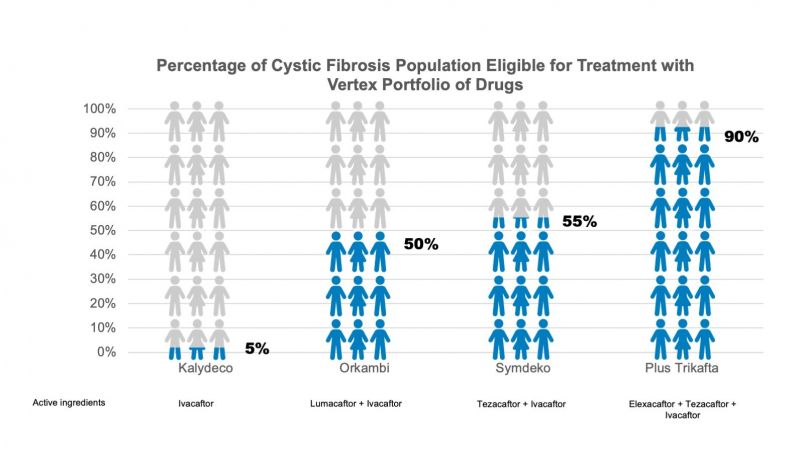
The Trikafta launch has been one of the most successful in the US, however, a proportion of sales has inevitably come from cannibalisation of their more established brands. Sales for Vertex's Kalydeco, Orkambi and Symdeko were down 13%, 20% and 46%, respectively following the launch of Trikafta (11).
It’s a bold move to launch a new brand that will primarily cannibalise the sales of a successful, patent protected portfolio of existing products, but one that’s been driven by Vertex’s commitment to the purpose set out by their CEO.
And at least half the company’s growth due to Trikafta has come from patients who weren’t previously taking a Vertex drug. In the US it seems that the majority of the 18,000 CF patients eligible to take Trikafta are now doing so (11).
Vertex hopes to further extend Trikafta’s eligibility criteria to include younger patients (11). New data demonstrate the brand’s efficacy and safety in children as young as six years (12) and will likely lead to Vertex extending its label accordingly, first in the US, and then elsewhere in the world.
Second – building long-standing, trusted relationships with patients

Source: Orkambi video (17)
Third – market access has been secured with innovative payer deals driven through by patient advocacy
There has, of course, been much controversy over the high list price of Vertex’s CF drugs (13), however, the prices are often discounted as part of innovative deals with payers driven through by patient advocacy.
In 2019 Vertex agreed plans with the Scottish government and NHS England to make Orkambi accessible to patients in the UK (14). This has continued with Trikafta being made available to CF patients in the UK from August 2020 (15). Together, these deals allow approximately 10,000 CF patients in the UK access to one of Vertex’s portfolio of CF drugs for free on the NHS.
As the UK makes up one of the largest populations of CF sufferers in the world (16), access is clearly of mutual benefit to both patients and company. Whilst there is little data on the exact terms of the deal, analysts predict that Vertex could earn between $420 million and $515 million per year from the UK market alone (16).
Payer support has been hard won, but driven through by the continued push of patient advocacy groups. Many will remember this clip of a 13-year-old patient finding out that Orkambi would be made available on the NHS. The video appeared widely across online news sites and reminds us forcibly of the huge human impact of innovative deals with payers such as the NHS.
Let’s remember what Trikafta offers
- Trikafta is a triple combination drug comprised of ivacaftor, elexacaftor and tezacaftor (6)
- Data including the results of two phase 3 clinical trials have led the Institute for Clinical and Economic Review (ICER) to conclude with “high certainty” that (for eligible patients) Trikafta is more effective than previous generation CF therapies. However, its list price is high at $311,000 per annum, and would need discounting by at least 73% in order to be cost-effective (13), (20)
- After its approval in the US, Trikafta contributed over 10% of Vertex 2019 sales despite only being on the market for 9 weeks (5)
- In the first quarter of 2020 it generated $895 million, a result almost 90% higher than the $474 million estimated by analysts (11)
- Trikafta is expected to generate $1.3 billion for Vertex in 2020 (21% revenue growth year-on-year) (5)
- Vertex Pharmaceuticals’ launch of Trikafta has been the 2nd most successful US drug launch in the past 5 years. (21)
Vertex’s expansion of their portfolio to cover a larger proportion of patients, combined with their current monopoly on therapies able to treat the underlying cause of the disease, is so compelling the company has effectively locked other players out of the market. Their dominance is making competition challenging and is one of the factors that has recently driven Savara to call it quits on their own CF program (10).
And let’s remember the promise it has fulfilled
The Trikafta launch has enabled Vertex to fulfil the promise the CEO made 9 years ago: the company does now offer a portfolio of drugs able to treat 90% of CF sufferers.
And the story continues – because perhaps that’s really the true power of purpose, that drive to continue – with the announcement in September 2020 of a new strategic research collaboration between Vertex and Moderna aimed at the discovery and development of lipid nanoparticles and mRNAs for the delivery of gene-editing therapies for the treatment of CF (22).
If you’d like to talk more about purposeful brands, whether in or outside of Pharma, please do get in touch – we’d love to hear from you.
Sources
1. FDA approves new breakthrough therapy for cystic fibrosis | FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-new-breakthrough-therapy-cystic-fibrosis. Accessed September 16, 2020.
2. “A game-changer”: How Vertex delivered on cystic fibrosis. https://www.statnews.com/2019/10/23/we-conquered-a-disease-how-vertex-delivered-a-transformative-medicine-for-cystic-fibrosis/. Accessed September 16, 2020.
3. Types of CFTR Mutations | CF Foundation. https://www.cff.org/What-is-CF/Genetics/Types-of-CFTR-Mutations/. Accessed September 16, 2020.
4. VRTX Stock Is a Biotech Play With No Coronavirus Exposure | InvestorPlace. https://investorplace.com/2020/06/vrtx-stock-is-a-biotech-play-with-no-coronavirus-exposure/. Accessed September 16, 2020.
5. Vertex Pharmaceuticals $VRTX Investment Case | LXV Research. https://lxvresearch.com/2020/03/31/vertex-pharmaceuticals-vrtx/. Accessed September 16, 2020.
6. Trikafta PRESCRIBING INFORMATION. https://pi.vrtx.com/files/uspi_elexacaftor_tezacaftor_ivacaftor.pdf. Accessed September 16, 2020.
7. SYMDEKO PRESCRIBING INFORMATION. https://pi.vrtx.com/files/uspi_tezacaftor_ivacaftor.pdf. Accessed September 16, 2020.
8. ORKAMBI PRESCRIBING INFORMATION. https://pi.vrtx.com/files/uspi_lumacaftor_ivacaftor.pdf. Accessed September 16, 2020.
9. KALYDECO PRESCRIBING INFORMATION. https://pi.vrtx.com/files/uspi_ivacaftor.pdf. Accessed September 16, 2020.
10. Vertex and Covid-19 spell the end for Savara’s CF program – Endpoints News. https://endpts.com/vertex-and-covid-19-spell-the-end-for-savaras-cf-program/. Accessed September 16, 2020.
11. Vertex’s drug launch hits new heights, but further growth could be harder to find | BioPharma Dive. https://www.biopharmadive.com/news/vertex-trikafta-drug-launch-new-heights-growth/577107/. Accessed September 16, 2020.
12. Vertex Pharmaceuticals Reports Positive Phase 3 Results in Children for Blockbuster CF Drug. https://www.fool.com/investing/2020/09/10/vertex-pharmaceuticals-reports-positive-phase-3-re/.
13. Trikafta Very Effective CF Therapy, But Still Too Costly, ICER Reports. https://cysticfibrosisnewstoday.com/2020/05/08/trikafta-very-effective-cf-therapy-but-still-too-costly-icer-reports/. Accessed September 16, 2020.
14. NHS England and Vertex break stalemate over price of CF drugs. https://www.pharmaceutical-technology.com/features/nhs-england-vertex-cf/. Accessed September 16, 2020.
15. NHS England » Landmark NHS deal to open up access to life-changing cystic fibrosis drug. https://www.england.nhs.uk/2020/08/landmark-nhs-deal-to-open-up-access-to-life-changing-cystic-fibrosis-drug/. Accessed September 16, 2020.
16. Vertex clears its biggest commercial hurdle: UK insurance coverage | BioPharma Dive. https://www.biopharmadive.com/news/vertex-england-reimbursement-deal-cystic-fibrosis-nhs/565749/. Accessed September 16, 2020.
17. “Overwhelming” response to Tamworth girl’s Orkambi news - BBC News. https://www.bbc.co.uk/news/uk-england-stoke-staffordshire-50181703. Accessed September 16, 2020.
18. Our History | CF Foundation. https://www.cff.org/About-Us/About-the-Cystic-Fibrosis-Foundation/Our-History/. Accessed September 16, 2020.
19. Cystic Fibrosis: Charity and Industry Partner for Profit | MedPage Today. https://www.medpagetoday.com/pulmonology/cysticfibrosis/39217. Accessed September 16, 2020.
20. ICER. Modulator Treatments for Cystic Fibrosis: Effectiveness and Value Evidence Report.; 2020. https://icer-review.org/wp-content/uploads/2019/09/ICER_CF_Evidence_Report_042720.pdf. Accessed September 16, 2020.
21. Vertex impresses with strong Trikafta launch | BioPharma Dive. https://www.biopharmadive.com/news/vertex-trikafta-launch-cystic-fibrosis-leiden/571452/. Accessed September 16, 2020.
22. Moderna and Vertex establish new collaboration to treat cystic fibrosis using gene editing | Businesswire. https://www.businesswire.com/n...;Accessed September 16, 2020.
Share this
You May Also Like
These Related Stories
Purpose, a route to regaining trust in Big Pharma

Closing the gap: the rise of prescription digital therapeutics

.png?width=657&height=57&name=OXFORD%20LOGO%20(1).png)
No Comments Yet
Let us know what you think